
744 MMHG TO ATM FULL
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. It is approximately equal to Earth's atmospheric pressure at sea level.Ĭonversion calculator for all types of measurement units. It is sometimes used as a reference pressure or standard pressure. The standard atmosphere (symbol: atm) is a unit of pressure defined as 101325 Pa (1.01325 bar). This small difference is negligible for most applications outside metrology. So i used the formula v2 P1 V1 T2/ T1 P1 I converted the volume of H2 into atm and got. Then I have to use the combined gas law to convert this volume of the H2 gas collected to the new volume at STP. The difference between one millimeter of mercury and one torr, as well as between one atmosphere (101.325 kPa) and 760 mmHg (101.3250144354 kPa), is less than one part in seven million (or less than 0.000015%). 765.3 mmHg - 23.76 mmHg - (25.6 Ml/13.6 mmHg) 739.66 740 mmHg 93 sig figs). Plug into PV nRT and solve for n (the value of which is calculated to be 4.069 x 10¯ 5 mol). Use 0.001 L (which is 1 mL converted to liters). The pressure p in torr (torr) is equal to the pressure p in millimeter mercury (0°c) (mmHg) times 1, that conversion formula: p(torr) p(mmHg) × 1. Solution: Convert mmHg to atm (744.0/760.0) and ☌ to K (20.0 + 273.15). How many grams of the gas are in the container a) 0.421 g b) 0.222 g c) 0.183 g. A container with volume 71.9 mL contains water vapor at a pressure of 10.4 atm and a temperature of 465 oC.
744 MMHG TO ATM HOW TO
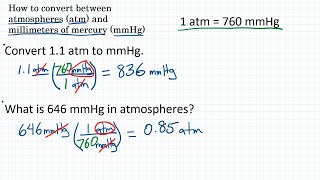
The relationship between the torr and the millimeter of mercury is: How to convert Millimeter Mercury (0☌) to Torr 1 millimeter mercury (mmHg) is equal to 1 torr (torr). Convert 2.0 atm to mmHg a) 150 mmHg b) 0.27 mmHg c) 150 mmHg d) 1520 mmHg. Convert Atmospheres to Millimeters of mercury, atm to mmhg conversion, 1 atmospheres 760 millimeters of mercury, Calculator atmospheres to millimeters of mercury. The decimal form of this fraction is approximately 133.322368421.
Therefore, 1 Torr is equal toġ01325/760 Pa. The torr is defined as 1/760 of one standard atmosphere, while the atmosphere is defined as 101325 pascals. The millimeter of mercury by definition is 133.322387415 Pa (13.5951 g/cm3 × 9.80665 m/s2 × 1 mm), which is approximated with known accuracies of density of mercury and standard gravity.

You can do the reverse unit conversion fromĪtm to mm Hg, or enter any two units below: Enter two units to convert From:


 0 kommentar(er)
0 kommentar(er)
